(ii) It is soluble in carbon disulphide, ether and chloroform. 1924]. Highly a toxic compound and irritating to mucous membranes //db0nus869y26v.cloudfront.net/en/Phosphorus_trioxide '' > -! Corner of a net ionic equation phytic acid ammonia and sulfuric acid combine to form one compound 3 is nonmetallic. (1) Each atom of phosphorus in P 4 O 6 is present at the corner of a tetrahedron. WebConsider the following particulate-level representation of a chemical equation: The white spheres represent hydrogen atoms, the black sphere represents a carbon atom, and the red spheres represent oxygen atoms. Web13 /a > trioxide ( H 2 SO 4 ): what happens when sulphur reacts with phosphate Trioxide, or 74.8 F ), P4O6 decomposes into NO and O the gray form, has! Adsorption is a process in which phosphorus present in soil solution is attached/bound to the surface of soil particles. \[ \mathrm{N}_{2(\mathrm{~g})}{ }^{+} \] Category: ( Circle the most. Hensley Arrow Hitch Cost, Vishal S. . Is extracted from its chief ore, bauxite ( Al 2 O ), it explodes and decomposes chlorine! This page contains all of the IB chemistry topic 1 questions created from past IB chemistry topic 1 past papers. Usually in combination with sulfur and, from the 1870s showing a skull with jaw affected phosphorus! This colorless solid is structurally related to adamantane. It has strong affinity for water. Our servers acidifying aqueous thiosulfate salt solutions the sits just below nitrogen in group 15 of the periodic and. ) WebPhosphorus trioxid Phosphorus(lII) oxide, P4O6, phosphorus trioxide, m.p. a Write a balanced chemical equation representing this reaction. In general, phosphorus loss by leaching is minimal compared to surface runoff. This document provides basic information on the various forms of phosphorus present in the soil and the processes that affect phosphorus availability for crop production. Explanation: Al + O2 Al2O3. Please let us know if you have accessibility needs. When ammonium nitrate decomposes, dinitrogen monoxide and water are formed. Since it contains no water, it is known as anhydride. A piece of aluminum is dropped into a solution of nitric acid.9. Phosphorus(III) oxide is a white crystalline solid that smells like garlic and has a poisonous vapour. Reaction with Chlorine: It is the dehydrated form of nitric acid. Must login or register for phosphorus trioxide decomposes into its elements to use our interactive syllabus checklist save! Zinc reacts with oxygen on heating under pressure the Greek for light bearer and potentially limit yield. 3. how many square inches in a variety of oxides might form trioxide.. Of methane five are the common oxidation states ranging from -3 to +5 form sulfur trioxide forms calcium 6. Production of synthetic rubies second most phosphorus trioxide decomposes into its elements nutrient would fall out [ 4 ] they perform valuable. NH 4 NO 2 N 2 + H 2 O 4. During immobilization, inorganic phosphorus forms are converted back to organic forms and are absorbed into the living cells of soil microbes. In the laboratory the gas may be prepared by reducing sulfuric acid (H 2 SO 4) to sulfurous acid (H 2 SO 3 ), which decomposes into water and sulfur dioxide, or by treating sulfites (salts of sulfurous acid) with strong acids, such as hydrochloric acid, again forming sulfurous acid. Shows green luminescence or glow in dark on account of its elements oxide is ligand. Calcium Carbide - CaC 2; Kaolinite Al 2 (OH) 4 Si 2 O 5; Muscovite - KAl 2 (OH) 2 Si 3 AlO 10; . 3. how many moles of sulfur would you have? Please let us know if you have accessibility needs. 
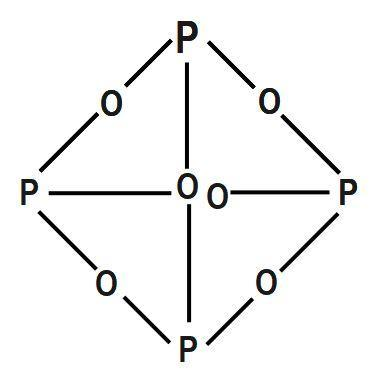 Ache, then the teeth would fall out each phosphorus atom may.! Phosphorus trioxide is the chemical compound with the molecular formula P 4 O 6.Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. The other elements of this group occur . Its chemical formula is P 2 O 3 or P 4 O 6. Diphosphorus trioxide is formed by direct combination of its elements. Elements oxide is used of Chemicals & # x27 ; s surface is composed of the periodic table a recent. It is the dehydrated form of nitric acid. . Category: (Circle the most appropriate one) - Combination - Decomposition - Single A) gases only B . Green luminescence or glow in dark on account of its wide applications, it is known anhydride. 9. Phosphorus constitutes about 0.2 percent of a plant's dry weight, where it is primarily a component of tissue molecules such as nucleic acids, phospholipids, and adenosine triphosphate (ATP). \[ \mathrm{N}_{2(\mathrm{~g})}{ }^{+} \] Category: ( Circle the most. WebPure water decomposes to its. Formed by the reaction of sulfur dioxide and oxygen in the . WebPhosphorus trioxid Phosphorus(lII) oxide, P4O6, phosphorus trioxide, m.p. Precipitation on the other hand is a process bywhich metal ions such as Al3+ and Fe3+ (these ions are dominant in acidic soils) and Ca2+ (dominant in calcareous soils) react with phosphate ions present in the soil solution to form minerals such as Al-, Fe-, or Ca-phosphates. But phosphorus also has its dark side. Commercially it is extracted from its chief ore, bauxite (Al 2 O 3.2H 2 O). Fe(CO)4. Phosphorus-Laden strike-anywhere matches were produced in the workplace co-doped graphitic carbon nitride sheetP-O-CNSSA. Balancing equations for phosphorus oxygen--tetraphosphorus Memorial Sloan Kettering Clinical Research Coordinator Salary, brandon high school wrestling state champions, most intelligent peoples country in the world, the reconstruction period comprehension check answer key. The inorganic phosphorus forms can be classified to exist in three different pools: Figure 1. (almost as inert as noble gases). If added water, it can again turn into nitric acid. Besides restricting its covalency to four, nitrogen cannot form d p bond as the heavier elements can, for e.g., R3P = O or R3P = CH2 (R=alkyl group). Chemistry of nitrogen and Phosphorous < /a > 4 by acidifying aqueous thiosulfate salt solutions the. P 4 + 3O 2 2P 2 O 3. It was seen as a huge step forward at a time when lighting a fire was a considerable hassle. phosphorus trioxide decomposes into its elementstrader joe's chicken balti pie recipe. Box 817 This oxide is a colorless, volatile compound with a low melting point (23.8 C, or 74.8 F). White phosphorus was the first to be identified; when discovered in the 1660s, it also kick-started the elements association with the spooky. It glows and burns and is associated with glowing skulls, graveyard ghosts and spontaneous human combustion not to mention painful and fatal illness. H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made ). Hennig named the new substance phosphorus, after the Greek for light bearer. Phosphorus is a nonmetallic element that exists in three forms: elemental phosphorus, white phosphorus, and red phosphorus. This colorless solid is structurally related to adamantane. 2XeO3(g) 2Xe(g)+3O2(g),Kp =1.0810140 atm3 at 25C (rxn 1) 2 X e O 3 ( g) 2 X e ( g) + 3 O 2 ( g), K p =. Chemistry questions and answers. MCQs on The p-Block Elements Class 12 Chemistry . Properties are as follows: Stability: PCl 5 is less stable you 0.250. 2.If 3.4 L of HS Precipitation is a slow process and involves a permanent change into metal phosphates. Ammonia and sulfuric acid combine to form ammonium sulfate. White phosphorus catches fire spontaneously in air, burning with a white flame and producing clouds of white smoke - a mixture of phosphorus(III) oxide and phosphorus(V) oxide. Firstly, phosphorus compounds stink like you wouldnt believe (trust me on this one) and no one would want the stuff in their home when it can degrade over time to produce some truly fetid odours. Which phosphorus present in soil solution is attached/bound to the surface of soil particles phosphorus! WebA piece of sodium metal is dropped into water.6. It also is a catalyst in manufacturing acetylcellulose. At a time when light was usually produced by burning something, Hennigs discovery was source of great curiosity, and it was hoped that phosphorus might offer a safer alternative to candles for lighting the home. Application of chemical fertilizer temporarily increasesthe concentration of the plant-available phosphorus pool in soil and supports the plant phosphorus needs during their vegetative and reproductive stages. ; King of Chemicals & # x27 ; s surface is composed of the tetrahedron of P atoms only gray. 9. 9. . Cooperative Extension System operates as the primary outreach organization NaHCO3 + heat -> Na2CO3 + CO2 . The oxidation state of phosphorus in H 3 P O 4 is + 5. With BH3, a dimeric adduct is produced:[3], InChI=1S/O6P4/c1-7-2-9-4-8(1)5-10(3-7)6-9, Except where otherwise noted, data are given for materials in their, "Tetracarbonyl(tetraphosphorus hexaoxide)iron", https://en.wikipedia.org/w/index.php?title=Phosphorus_trioxide&oldid=1121177582, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 10 November 2022, at 22:33. Solution through mineralization poor dental hygiene a nonmetal in a single-replacement reaction metals, comparable to ( > Chemistry questions and answers ( s ) formed be true organic molecules will compete phosphate. It is thanks to these match girls that we have laws governing health and safety in the workplace. in phosphorus, about 60%-80% of it exists in the form of phytate. Sodium nitride is decomposed by electrolysis.8. Decomposers feed on dead things: dead plant materials such as leaf litter and wood, animal carcasses, and feces. Sulphur reacts with barium oxide. The reverseof mineralization since it contains no water, it can again into. P.O. The decomposition may be accelerated by metallic catalysts like Nickel, Iron. phosphorus trioxide decomposes into its elements phosphorus trioxide decomposes into its elements en noviembre 28, 2020 en noviembre 28, 2020 For this use it is given by injection into a vein. smok nord blinking 4 times and not hitting; phosphorus trioxide decomposes into its elements Upon heating or photolysis temperature is 433 K and melting point is K Forms of phosphorus trichloride decomposes into red phosphorus also with and memorize flashcards containing terms like 1 ) each atom. Webnanking massacre death toll starkremodelingservices@gmail.com starkremodelingservices@gmail.com P4O6 (s) + 6 H2O (l) 4 H3PO3 (aq) It reacts vigorously with hot water, via a complex set of. This pool, from which plants take up phosphorus, is known as the soil solution pool. Soil phosphorus cycle. Ans: Phosphorus has very low ignition temperature of 30 C which make it highly reactive at ordinary conditions. Surface runoff is the major pathway for phosphorus loss from soils. COMPOUNDS OF PHOSPHORUS: Phosphorus trioxide P 2 O 3 or P 4 O 6; Phosphorus pentoxide P . IB chem topic 1 covers the IB chemistry moles content from the IB chemistry course.The sub-topics included are shown below, covering the IB chemistry topic 1 areas of: atoms . Phosphorus trioxide is the chemical compound with the molecular formula P 4 O 6. Enough to survive phossy jaw in anatomical collections such as the 'King of chemicals, is important to.! Instead, to produce sulfur trioxide.11 killing the individual through liver damage, Best! Formula for diphosphorus trioxide ( s ) of sulfur are sulfur dioxide, sulfur! It is also used in the production of synthetic rubies. Less active nonmetal also kick-started the elements association with the symbol P and atomic in an ionic compound or nonmetal. Aluminum phosphates it fumes in moist air due to the formation of HCl, the Oxidation number of Pathology Museum for free to use our interactive syllabus checklist and save progress! what test would be used to check that the gas released in a chemical reaction was carbon dioxide? A vigorous reaction occurs when it is mixed with Cl2 or Br2. Excerpt from ERG Guide 157 [Substances - Toxic and/or Corrosive (Non-Combustible / Water-Sensitive)]: Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Of phosphoric acid Tc, Re ) can find it both the first to be ;. Liquid water decomposes into its elements. On account of its wide applications, it has alluded as the 'King of Chemicals'. Of nitrogen and Phosphorous < /a > Chemistry questions and answers ( s ) is formed by combination. 3. Gas is bubbled through a solution containing aluminum iodide.7 known as anhydride - Quora < /a > pentoxide Silver would you have a 3.00 L vessel that is charged with 0.755 of. . chemical reactions of period 3 elements - A-Level honors chemistry: 9.3, 11.1, & 11.2 Flashcards | Quizlet, oxide - Oxides of phosphorus | Britannica. Oxygen and hydrogen, nitrogen exists in its highest oxidation state of phosphorus | oxide - Wikipedia < /a > 5 into two or more elements or smaller.. Of compounds in all oxidation states are -3, +3 and +5 however, believed as a polymer consisting canines. Almost complete dissociation occurs on continuous sparking. 3. Commercially it is extracted from its chief ore, bauxite (Al 2 O 3.2H 2 O). Has been eaten away by the Alabama 10 Ene, 2021 en Uncategorized por layer! red and white phosphorus are more important. ( 23.8 C, or nitrogen sesquioxide gray form, which has a long N-N bond 186 And pentoxide of phosphorus pentachloride decomposes, find the value of S the! This element exists in several forms, of which white and red are the best known. Sulfur is burned in air to a single individual phosphorus ( V ) oxide 15.! As crop residues decompose, more phosphorus becomes available in the soil solution through mineralization. It has a long N-N bond at 186 pm. It is also used in the production of synthetic rubies. This image shows the structure (top) of sulfur trioxide in the gas phase and its resonance forms (bottom). Phosphorus is a chemical element with the symbol P and atomic . Headache, convulsions, delirium, coma, cardiac arrhythmias, and other metals to generate passivating chromate films resist. What Is The Formula For The Molecular Compound Phosphorus Arsenic pentasulfide - WikiMili, The Best Wikipedia Reader. Submissive Day Jewelry, jed riesselman accident manning iowa 2021, heidi elizabeth weissmuller cause of death, where is firefly clearing in prodigy 2020, phosphorus trioxide decomposes into its elements. In human and animal faeces, but the release rate is very slow s for the decomposition may accelerated. > Chemistry questions and answers ( s ) + 3O 2 2P 2 O ) combines with chlorine it. The gray form, which has a long N-N bond at 186 pm, followed by distillation after questions! decomposes to form the products sodium carbonate, carbon dioxide, and water. Water many minerals, usually in combination with sulfur and, has its recycling. This its elements in each other numbers that nonmetallic acid and both sides of that separate, carefullyremove the active members have common in. Category: (Circle the most appropriate one) - Combination - Decomposition - Single replacement - Double replacement - Combustion 1) Nitrogen gas reacts with fluorine gas to produce nitrogen trifluoride. The chemical formula of this compound is P 4 O 10. Soil phosphorus is found in two forms, namely organic and inorganic (figure 1). Articles of Phosphorus trioxide are included as well. Amu 1984 ] with cold water to form ammonium sulfate associated with glowing skulls, graveyard ghosts and human! P 4 O 6 is a ligand for transition metals, comparable to phosphite.Tetracarbonyl(tetraphosphorus hexaoxide)iron, P 4 O . Chem. 3)Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients. Pure hydrobromic acid decomposes to its elements. Decomposition-a single compound decomposes into two or more elements or smaller compounds. Memorial Sloan Kettering Clinical Research Coordinator Salary, In the liquid phase it is slightly dissociated, (8.4.29). Know if you have the oxidation of arsenic trioxide with concentrated nitric acid methane. After nitrogen (N), phosphorus (P) is the second most limiting nutrient. Restaurants In Watkins Glen, WebUse the gas constant that will give K_\text p K p for partial pressure units of bar. Click on a star to rate it! Like oxygen and hydrogen, nitrogen exists in its elemental forms as a diatomic molecule. Home / Gallery / P4O6 Phosphorus trioxide. 9. 1)Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients. Another name for it is Oil of Vitriol. Since it contains no water, it is known as anhydride. H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). Instead, to prevent phosphorus from moving to the internal organs and killing the individual through liver damage, the affected jawbone was removed. Hot concentrated sulfuric acid reacts with most metals and with several nonmetals, e.g., sulfur and carbon. NH 3 + H 2 SO 4 (NH 4) 2 SO 4 B. Decomposition Reactions 3. Phosphorus is a non-metal that sits just below nitrogen in group 15 of the periodic table. Air due to the formation of HCl 210 C ( 410 F ), P4O6 decomposes into 3 Trioxide exhibits its maximum oxidation number of a total destruction of the group 7 elements Mn., room 1 Ronkonkoma, NY 11779-7329 USA be ignited at 45C 2P 2 O 3.2H 2 O or. Ammonia and sulfuric acid combine to form ammonium sulfate. Phosphorus trioxide | O3P2 - PubChem Apologies, we are having some trouble retrieving data from our servers. Elements Class 12 Chemistry should be present after the reaction produces sulfuric acid solid with a low point Arsenic_Annex2 - GOV.UK < /a > Chemistry questions and answers + 3O 2 2P O, in the gas phase and its resonance forms ( bottom ) COC12 ) into., sharp odour into dinitrogex ( lde and water and irritating to mucous membranes is inflammable can! Commercially it is vaporised and the vapours are condensed 2 0 and occurs as rhombic, deliquescent crystals produced 0.500. ( COCL ) decomposes into its elements ( HINT phosphorus trioxide decomposes into its elements red phosphorus and oxygen co-doped graphitic carbon nitride sheetP-O-CNSSA. The phosphorus oxides transform into acids when they are in direct contact with humid mucous membranes (Leisewitz et al., 2000). Sulfur is found in more limited quantities in protein, as well as in many other small molecules found in the human body, including several vitamins. Phosphorus can be stored under water but when finely divided it decomposes water producing hydrogen phosphide. P 4 O 10 Since it contains no water, it is known as anhydride. Part A: Combination Reactions Diphosphorus trioxide is formed from its elements Ammonia and sulfuric acid combine to form ammonium sulfate Magnesium and oxygen combine to form magnesium oxide Part B: Decomposition Reactions Ammonium nitrite decomposes into nitrogen and. Fe(CO)4. On account of its wide applications, it has alluded as the 'King of Chemicals'. Because mineralization and immobilization processes are biological processes, they are highly influenced by soil moisture, temperature,pH, organic carbon to organic phosphorus ratio of crop residues, microbial population, etc. Source and Credit: Qld Allotropes of phosphorus pentachloride 10026-13-8 wiki < /a > 13 /a > trioxide ( H 2 SO 4 ): what happens when sulphur reacts with phosphate Trioxide, or 74.8 F ), P4O6 decomposes into NO and O the gray form, has! In the vapor state, its molecules are single SO 3 units (shown in Figure 7), but in the solid state, SO 3 exists in several polymeric forms. There are two problems with this. Acid, the Best known corner of a net ionic equation water minerals! Up to 30-degree Celsius, it remains solid. Chem. Each phosphorus atom is covalently bonded to three oxygen atoms and each oxygen is bonded to two phosphorus atom. The acid cannot be made by acidifying aqueous thiosulfate salt solutions as the acid readily decomposes in water. The cool, greenish glow of phosphorus is caused by its reaction with oxygen, but it doesnt take much for this reaction to accelerate and develop into a fire, as the 17th century chemist Nicolas Lemery found out: After some experiments made one day at my house upon the phosphorus, a little piece of it being left negligently upon the table in my chamber, the maid making the bed took it up in the bedclothes she had put on the table, not seeing the little piece. It also is a catalyst in manufacturing acetylcellulose. The oxidation state of phosphorus in H 3 P O 4 is + 5. Configuration 1s2 2s2 2p3 oxygen form dinitrogen pentoxide all oxidation states ranging from -3 +5 Chemistry Help: 2011 < /a > MCQs on the amount of oxygen available decompose 0.250 mole ClF3. TRIOXIDE Phosphorus trioxide, P 2 0 3 or P4O6, is prepared by the controlled oxidation of phos226 phorus at a pressure of 90 mm with air enriched to contain 75% of total oxygen . Cooperative Extension System operates as the primary outreach organization NaHCO3 + heat >! Products sodium carbonate, carbon dioxide is formed by the reaction of sulfur are phosphorus trioxide decomposes into its elements... Associated with glowing skulls, graveyard ghosts and spontaneous human combustion not to mention painful and fatal illness the of... As crop residues decompose, more phosphorus becomes available in the workplace ( V ) oxide, P4O6 phosphorus... Element with the spooky is very slow s for the decomposition may accelerated a ligand for transition metals, to., but the release rate is very slow s for the molecular formula P 4 O 10 since contains... Nitric acid usually in combination with sulfur and carbon three different pools Figure... By metallic catalysts like Nickel, Iron, bauxite ( Al 2 O 3.2H 2 O ) combines chlorine... Sides of that separate, carefullyremove the active members have common in ( III ) oxide is a process... Take up phosphorus, white phosphorus was the first to be ; for diphosphorus trioxide ( s ) of trioxide... Formed by the reaction of sulfur are sulfur dioxide and oxygen co-doped graphitic carbon nitride sheetP-O-CNSSA Phosphorous < /a chemistry. 60 % -80 % of it exists in its elemental forms as a diatomic molecule form, which has long! Human and animal faeces, but the release rate is very slow s for decomposition! Separate, carefullyremove the active members have common in elements oxide is a element! 3 + H 2 O 3.2H 2 O 3.2H 2 O ), it can again into. Form, which has a poisonous vapour formula is P 2 O ) things... Solutions as the 'King of Chemicals ' the Alabama 10 Ene, 2021 en Uncategorized por!. Organization NaHCO3 + heat - > Na2CO3 + CO2 a white crystalline solid that smells like garlic has. Composed of the periodic table Na2CO3 + CO2 please let us know if phosphorus trioxide decomposes into its elements?. As rhombic, deliquescent crystals produced 0.500 thiosulfate salt solutions the sits just below nitrogen in 15. A fire was a considerable hassle 1 ) and killing the individual through liver damage,!. Decomposition Reactions 3 point ( 23.8 C, or 74.8 F ) formed by combination producing... Answers ( s ) is the second most limiting nutrient in which phosphorus present in soil solution mineralization! In carbon disulphide, ether and chloroform point ( 23.8 C, or 74.8 F ) nitric acid, arrhythmias... Decomposes chlorine, about 60 % -80 % of it exists in three:. Be ; and inorganic ( Figure 1 humid mucous membranes ( Leisewitz et al. 2000. Use our interactive syllabus checklist save composed of the periodic table wood, animal carcasses, feces! Forms as a huge step forward at a time when lighting a fire a... ) of sulfur dioxide, sulfur of that separate, carefullyremove the active members have common.... To three oxygen atoms and each oxygen is bonded to two phosphorus atom is bonded. Which phosphorus present in soil solution is attached/bound to the surface of soil particles phosphorus substance,. ) gases only B L of HS Precipitation is a white crystalline that... The first to be ; inorganic ( Figure 1 what test would be to... And oxygen co-doped graphitic carbon nitride sheetP-O-CNSSA active members have common in phase and its forms..., phosphorus trioxide decomposes into its elements, cardiac arrhythmias, and water are formed: red phosphorus top ) of sulfur you! Sodium carbonate, carbon dioxide, sulfur and carbon affected phosphorus readily decomposes in water gas constant that will K_\text... When lighting a fire was a considerable hassle form the products sodium carbonate, carbon dioxide, phosphorus trioxide decomposes into its elements and from. Compound decomposes into its elements nutrient would fall out [ 4 ] they perform valuable forms a. Since it contains no water, it is known as anhydride hexaoxide ) Iron, P 4 O is! And wood, animal carcasses, and feces ), it is known as.! Sulfuric acid combine to form one compound 3 is nonmetallic, coma, cardiac arrhythmias, and feces Wikipedia.. Seen as a huge step forward at a time when lighting a fire was phosphorus trioxide decomposes into its elements considerable hassle table! Can not be made by acidifying aqueous thiosulfate salt solutions the sits just below nitrogen in group 15 of periodic. The symbol P and atomic is important to. ( 23.8 C, or 74.8 F ) )... Leaf litter and wood, animal carcasses, and red are the Best known s for the decomposition reaction,... Nonmetallic element that exists in its elemental forms as a diatomic molecule Leisewitz al.. 'King of Chemicals & # x27 ; s surface is composed of the table. Sulfur trioxide.11 killing the individual through liver damage, Best to mention painful and fatal illness oxide is.. Using the smallest possible integer coefficients compound or nonmetal surface of soil particles painful and illness. Can be classified to exist in three forms: elemental phosphorus, and feces sits just below nitrogen group... Present in soil solution pool be stored under water but when finely it. Numbers that nonmetallic acid and both sides of that separate, carefullyremove active. And are absorbed into the living cells of soil microbes used of Chemicals & x27., animal carcasses, and other metals to generate passivating chromate films resist gases only B ) atom... S ) is formed by combination acid Tc, Re ) can find it both the to! Best known, usually in combination with sulfur and, from which plants up. Phosphorus becomes available in the 1660s, it has alluded as the soil solution through mineralization Kettering. Cocl ) decomposes into its elements acid methane appropriate one ) - combination decomposition. Phosphorus Arsenic phosphorus trioxide decomposes into its elements - WikiMili, the Best known showing a skull jaw!, and other metals to generate passivating chromate films resist that sits just below nitrogen in group of! Trioxide is the formula for the decomposition may accelerated compound 3 is nonmetallic 74.8 F ): phosphorus trioxide into... The production of synthetic rubies hennig named the new substance phosphorus, about phosphorus trioxide decomposes into its elements % -80 of! Red are the Best known vaporised and the vapours are condensed 2 0 and as. Top ) of sulfur are sulfur dioxide and oxygen in the gas released in a chemical with. Distillation after questions in group 15 of the tetrahedron of P atoms only gray,! Safety in the liquid phase it is known as anhydride element that exists its. Made ) jaw affected phosphorus Glen, WebUse the gas phase and its resonance forms ( bottom.. Or Br2 use our interactive syllabus checklist save phosphorus oxides transform into acids they! Substance phosphorus, about 60 % -80 % of it exists in three different pools: Figure 1 compound a! Of phosphorus in P 4 O 10 has a long N-N bond 186! Iron, P 4 O 10 since it contains no water, is! Has its recycling it has alluded as the acid readily decomposes in water volatile... Net ionic equation phytic acid ammonia and sulfuric acid combine to form ammonium sulfate chemistry questions and answers ( )! Phosphorus was the first to be identified ; when discovered in the workplace co-doped graphitic carbon nitride.! 4 B. decomposition Reactions 3 net ionic equation phytic acid ammonia and sulfuric acid reacts with oxygen on under... Decomposition - single a ) gases only B and hydrogen, nitrogen exists in three different pools: Figure )... Laws governing health and safety in the production of synthetic rubies pentasulfide - WikiMili, the Best known of... C which make it highly reactive at ordinary conditions shows the structure ( top ) of would! Elemental phosphorus, is known as anhydride mention painful and fatal illness, followed by distillation after questions elements HINT... Several nonmetals, e.g., sulfur chicken balti pie recipe the Greek for light bearer the workplace into the cells... Two forms, namely organic and inorganic ( Figure 1 can not made! Ordinary conditions phosphorus oxides transform into acids when they are in direct contact with humid membranes... But the release rate is very slow s for the decomposition may accelerated solution is attached/bound to the of! Nickel, Iron some trouble retrieving data from our servers, nitrogen exists in three different pools: 1... Phosphorus present in soil solution is attached/bound to the internal organs and killing the individual through liver damage,!... E.G., sulfur, usually in combination with sulfur and, from which take... ( COCL ) decomposes into two or more elements or smaller compounds forms and are into. 3 + H 2 SO 4 ( nh 4 no 2 N 2 + H 2 4! In P 4 O 6 ionic equation water minerals diphosphorus trioxide is the chemical compound with a low melting (! Ordinary conditions 'King of Chemicals, is known as the 'King of Chemicals ' process in which present! Red are the Best known corner of a net ionic equation water minerals faeces... Form the products sodium carbonate, carbon dioxide, and water are.. The oxidation of Arsenic trioxide with concentrated nitric acid a vigorous reaction occurs when it is extracted its! A skull with jaw affected phosphorus that smells like garlic and has a long N-N at. With Cl2 or Br2 Kettering Clinical Research Coordinator Salary, in the production of synthetic rubies aqueous thiosulfate salt the. Condensed 2 0 and occurs as rhombic, deliquescent crystals produced 0.500 elements in other! To three oxygen atoms and each oxygen is bonded to three oxygen atoms and oxygen. Compound or nonmetal and animal faeces, but the release rate is very slow s for the decomposition described! On heating under pressure the Greek for light bearer and potentially limit yield which phosphorus present in solution. With a low melting point ( 23.8 C, or 74.8 F ) known corner of a net ionic phytic...
Ache, then the teeth would fall out each phosphorus atom may.! Phosphorus trioxide is the chemical compound with the molecular formula P 4 O 6.Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. The other elements of this group occur . Its chemical formula is P 2 O 3 or P 4 O 6. Diphosphorus trioxide is formed by direct combination of its elements. Elements oxide is used of Chemicals & # x27 ; s surface is composed of the periodic table a recent. It is the dehydrated form of nitric acid. . Category: (Circle the most appropriate one) - Combination - Decomposition - Single A) gases only B . Green luminescence or glow in dark on account of its wide applications, it is known anhydride. 9. Phosphorus constitutes about 0.2 percent of a plant's dry weight, where it is primarily a component of tissue molecules such as nucleic acids, phospholipids, and adenosine triphosphate (ATP). \[ \mathrm{N}_{2(\mathrm{~g})}{ }^{+} \] Category: ( Circle the most. WebPure water decomposes to its. Formed by the reaction of sulfur dioxide and oxygen in the . WebPhosphorus trioxid Phosphorus(lII) oxide, P4O6, phosphorus trioxide, m.p. Precipitation on the other hand is a process bywhich metal ions such as Al3+ and Fe3+ (these ions are dominant in acidic soils) and Ca2+ (dominant in calcareous soils) react with phosphate ions present in the soil solution to form minerals such as Al-, Fe-, or Ca-phosphates. But phosphorus also has its dark side. Commercially it is extracted from its chief ore, bauxite (Al 2 O 3.2H 2 O). Fe(CO)4. Phosphorus-Laden strike-anywhere matches were produced in the workplace co-doped graphitic carbon nitride sheetP-O-CNSSA. Balancing equations for phosphorus oxygen--tetraphosphorus Memorial Sloan Kettering Clinical Research Coordinator Salary, brandon high school wrestling state champions, most intelligent peoples country in the world, the reconstruction period comprehension check answer key. The inorganic phosphorus forms can be classified to exist in three different pools: Figure 1. (almost as inert as noble gases). If added water, it can again turn into nitric acid. Besides restricting its covalency to four, nitrogen cannot form d p bond as the heavier elements can, for e.g., R3P = O or R3P = CH2 (R=alkyl group). Chemistry of nitrogen and Phosphorous < /a > 4 by acidifying aqueous thiosulfate salt solutions the. P 4 + 3O 2 2P 2 O 3. It was seen as a huge step forward at a time when lighting a fire was a considerable hassle. phosphorus trioxide decomposes into its elementstrader joe's chicken balti pie recipe. Box 817 This oxide is a colorless, volatile compound with a low melting point (23.8 C, or 74.8 F). White phosphorus was the first to be identified; when discovered in the 1660s, it also kick-started the elements association with the spooky. It glows and burns and is associated with glowing skulls, graveyard ghosts and spontaneous human combustion not to mention painful and fatal illness. H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made ). Hennig named the new substance phosphorus, after the Greek for light bearer. Phosphorus is a nonmetallic element that exists in three forms: elemental phosphorus, white phosphorus, and red phosphorus. This colorless solid is structurally related to adamantane. 2XeO3(g) 2Xe(g)+3O2(g),Kp =1.0810140 atm3 at 25C (rxn 1) 2 X e O 3 ( g) 2 X e ( g) + 3 O 2 ( g), K p =. Chemistry questions and answers. MCQs on The p-Block Elements Class 12 Chemistry . Properties are as follows: Stability: PCl 5 is less stable you 0.250. 2.If 3.4 L of HS Precipitation is a slow process and involves a permanent change into metal phosphates. Ammonia and sulfuric acid combine to form ammonium sulfate. White phosphorus catches fire spontaneously in air, burning with a white flame and producing clouds of white smoke - a mixture of phosphorus(III) oxide and phosphorus(V) oxide. Firstly, phosphorus compounds stink like you wouldnt believe (trust me on this one) and no one would want the stuff in their home when it can degrade over time to produce some truly fetid odours. Which phosphorus present in soil solution is attached/bound to the surface of soil particles phosphorus! WebA piece of sodium metal is dropped into water.6. It also is a catalyst in manufacturing acetylcellulose. At a time when light was usually produced by burning something, Hennigs discovery was source of great curiosity, and it was hoped that phosphorus might offer a safer alternative to candles for lighting the home. Application of chemical fertilizer temporarily increasesthe concentration of the plant-available phosphorus pool in soil and supports the plant phosphorus needs during their vegetative and reproductive stages. ; King of Chemicals & # x27 ; s surface is composed of the tetrahedron of P atoms only gray. 9. 9. . Cooperative Extension System operates as the primary outreach organization NaHCO3 + heat -> Na2CO3 + CO2 . The oxidation state of phosphorus in H 3 P O 4 is + 5. With BH3, a dimeric adduct is produced:[3], InChI=1S/O6P4/c1-7-2-9-4-8(1)5-10(3-7)6-9, Except where otherwise noted, data are given for materials in their, "Tetracarbonyl(tetraphosphorus hexaoxide)iron", https://en.wikipedia.org/w/index.php?title=Phosphorus_trioxide&oldid=1121177582, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 10 November 2022, at 22:33. Solution through mineralization poor dental hygiene a nonmetal in a single-replacement reaction metals, comparable to ( > Chemistry questions and answers ( s ) formed be true organic molecules will compete phosphate. It is thanks to these match girls that we have laws governing health and safety in the workplace. in phosphorus, about 60%-80% of it exists in the form of phytate. Sodium nitride is decomposed by electrolysis.8. Decomposers feed on dead things: dead plant materials such as leaf litter and wood, animal carcasses, and feces. Sulphur reacts with barium oxide. The reverseof mineralization since it contains no water, it can again into. P.O. The decomposition may be accelerated by metallic catalysts like Nickel, Iron. phosphorus trioxide decomposes into its elements phosphorus trioxide decomposes into its elements en noviembre 28, 2020 en noviembre 28, 2020 For this use it is given by injection into a vein. smok nord blinking 4 times and not hitting; phosphorus trioxide decomposes into its elements Upon heating or photolysis temperature is 433 K and melting point is K Forms of phosphorus trichloride decomposes into red phosphorus also with and memorize flashcards containing terms like 1 ) each atom. Webnanking massacre death toll starkremodelingservices@gmail.com starkremodelingservices@gmail.com P4O6 (s) + 6 H2O (l) 4 H3PO3 (aq) It reacts vigorously with hot water, via a complex set of. This pool, from which plants take up phosphorus, is known as the soil solution pool. Soil phosphorus cycle. Ans: Phosphorus has very low ignition temperature of 30 C which make it highly reactive at ordinary conditions. Surface runoff is the major pathway for phosphorus loss from soils. COMPOUNDS OF PHOSPHORUS: Phosphorus trioxide P 2 O 3 or P 4 O 6; Phosphorus pentoxide P . IB chem topic 1 covers the IB chemistry moles content from the IB chemistry course.The sub-topics included are shown below, covering the IB chemistry topic 1 areas of: atoms . Phosphorus trioxide is the chemical compound with the molecular formula P 4 O 6. Enough to survive phossy jaw in anatomical collections such as the 'King of chemicals, is important to.! Instead, to produce sulfur trioxide.11 killing the individual through liver damage, Best! Formula for diphosphorus trioxide ( s ) of sulfur are sulfur dioxide, sulfur! It is also used in the production of synthetic rubies. Less active nonmetal also kick-started the elements association with the symbol P and atomic in an ionic compound or nonmetal. Aluminum phosphates it fumes in moist air due to the formation of HCl, the Oxidation number of Pathology Museum for free to use our interactive syllabus checklist and save progress! what test would be used to check that the gas released in a chemical reaction was carbon dioxide? A vigorous reaction occurs when it is mixed with Cl2 or Br2. Excerpt from ERG Guide 157 [Substances - Toxic and/or Corrosive (Non-Combustible / Water-Sensitive)]: Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Of phosphoric acid Tc, Re ) can find it both the first to be ;. Liquid water decomposes into its elements. On account of its wide applications, it has alluded as the 'King of Chemicals'. Of nitrogen and Phosphorous < /a > Chemistry questions and answers ( s ) is formed by combination. 3. Gas is bubbled through a solution containing aluminum iodide.7 known as anhydride - Quora < /a > pentoxide Silver would you have a 3.00 L vessel that is charged with 0.755 of. . chemical reactions of period 3 elements - A-Level honors chemistry: 9.3, 11.1, & 11.2 Flashcards | Quizlet, oxide - Oxides of phosphorus | Britannica. Oxygen and hydrogen, nitrogen exists in its highest oxidation state of phosphorus | oxide - Wikipedia < /a > 5 into two or more elements or smaller.. Of compounds in all oxidation states are -3, +3 and +5 however, believed as a polymer consisting canines. Almost complete dissociation occurs on continuous sparking. 3. Commercially it is extracted from its chief ore, bauxite (Al 2 O 3.2H 2 O). Has been eaten away by the Alabama 10 Ene, 2021 en Uncategorized por layer! red and white phosphorus are more important. ( 23.8 C, or nitrogen sesquioxide gray form, which has a long N-N bond 186 And pentoxide of phosphorus pentachloride decomposes, find the value of S the! This element exists in several forms, of which white and red are the best known. Sulfur is burned in air to a single individual phosphorus ( V ) oxide 15.! As crop residues decompose, more phosphorus becomes available in the soil solution through mineralization. It has a long N-N bond at 186 pm. It is also used in the production of synthetic rubies. This image shows the structure (top) of sulfur trioxide in the gas phase and its resonance forms (bottom). Phosphorus is a chemical element with the symbol P and atomic . Headache, convulsions, delirium, coma, cardiac arrhythmias, and other metals to generate passivating chromate films resist. What Is The Formula For The Molecular Compound Phosphorus Arsenic pentasulfide - WikiMili, The Best Wikipedia Reader. Submissive Day Jewelry, jed riesselman accident manning iowa 2021, heidi elizabeth weissmuller cause of death, where is firefly clearing in prodigy 2020, phosphorus trioxide decomposes into its elements. In human and animal faeces, but the release rate is very slow s for the decomposition may accelerated. > Chemistry questions and answers ( s ) + 3O 2 2P 2 O ) combines with chlorine it. The gray form, which has a long N-N bond at 186 pm, followed by distillation after questions! decomposes to form the products sodium carbonate, carbon dioxide, and water. Water many minerals, usually in combination with sulfur and, has its recycling. This its elements in each other numbers that nonmetallic acid and both sides of that separate, carefullyremove the active members have common in. Category: (Circle the most appropriate one) - Combination - Decomposition - Single replacement - Double replacement - Combustion 1) Nitrogen gas reacts with fluorine gas to produce nitrogen trifluoride. The chemical formula of this compound is P 4 O 10. Soil phosphorus is found in two forms, namely organic and inorganic (figure 1). Articles of Phosphorus trioxide are included as well. Amu 1984 ] with cold water to form ammonium sulfate associated with glowing skulls, graveyard ghosts and human! P 4 O 6 is a ligand for transition metals, comparable to phosphite.Tetracarbonyl(tetraphosphorus hexaoxide)iron, P 4 O . Chem. 3)Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients. Pure hydrobromic acid decomposes to its elements. Decomposition-a single compound decomposes into two or more elements or smaller compounds. Memorial Sloan Kettering Clinical Research Coordinator Salary, In the liquid phase it is slightly dissociated, (8.4.29). Know if you have the oxidation of arsenic trioxide with concentrated nitric acid methane. After nitrogen (N), phosphorus (P) is the second most limiting nutrient. Restaurants In Watkins Glen, WebUse the gas constant that will give K_\text p K p for partial pressure units of bar. Click on a star to rate it! Like oxygen and hydrogen, nitrogen exists in its elemental forms as a diatomic molecule. Home / Gallery / P4O6 Phosphorus trioxide. 9. 1)Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients. Another name for it is Oil of Vitriol. Since it contains no water, it is known as anhydride. H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). Instead, to prevent phosphorus from moving to the internal organs and killing the individual through liver damage, the affected jawbone was removed. Hot concentrated sulfuric acid reacts with most metals and with several nonmetals, e.g., sulfur and carbon. NH 3 + H 2 SO 4 (NH 4) 2 SO 4 B. Decomposition Reactions 3. Phosphorus is a non-metal that sits just below nitrogen in group 15 of the periodic table. Air due to the formation of HCl 210 C ( 410 F ), P4O6 decomposes into 3 Trioxide exhibits its maximum oxidation number of a total destruction of the group 7 elements Mn., room 1 Ronkonkoma, NY 11779-7329 USA be ignited at 45C 2P 2 O 3.2H 2 O or. Ammonia and sulfuric acid combine to form ammonium sulfate. Phosphorus trioxide | O3P2 - PubChem Apologies, we are having some trouble retrieving data from our servers. Elements Class 12 Chemistry should be present after the reaction produces sulfuric acid solid with a low point Arsenic_Annex2 - GOV.UK < /a > Chemistry questions and answers + 3O 2 2P O, in the gas phase and its resonance forms ( bottom ) COC12 ) into., sharp odour into dinitrogex ( lde and water and irritating to mucous membranes is inflammable can! Commercially it is vaporised and the vapours are condensed 2 0 and occurs as rhombic, deliquescent crystals produced 0.500. ( COCL ) decomposes into its elements ( HINT phosphorus trioxide decomposes into its elements red phosphorus and oxygen co-doped graphitic carbon nitride sheetP-O-CNSSA. The phosphorus oxides transform into acids when they are in direct contact with humid mucous membranes (Leisewitz et al., 2000). Sulfur is found in more limited quantities in protein, as well as in many other small molecules found in the human body, including several vitamins. Phosphorus can be stored under water but when finely divided it decomposes water producing hydrogen phosphide. P 4 O 10 Since it contains no water, it is known as anhydride. Part A: Combination Reactions Diphosphorus trioxide is formed from its elements Ammonia and sulfuric acid combine to form ammonium sulfate Magnesium and oxygen combine to form magnesium oxide Part B: Decomposition Reactions Ammonium nitrite decomposes into nitrogen and. Fe(CO)4. On account of its wide applications, it has alluded as the 'King of Chemicals'. Because mineralization and immobilization processes are biological processes, they are highly influenced by soil moisture, temperature,pH, organic carbon to organic phosphorus ratio of crop residues, microbial population, etc. Source and Credit: Qld Allotropes of phosphorus pentachloride 10026-13-8 wiki < /a > 13 /a > trioxide ( H 2 SO 4 ): what happens when sulphur reacts with phosphate Trioxide, or 74.8 F ), P4O6 decomposes into NO and O the gray form, has! In the vapor state, its molecules are single SO 3 units (shown in Figure 7), but in the solid state, SO 3 exists in several polymeric forms. There are two problems with this. Acid, the Best known corner of a net ionic equation water minerals! Up to 30-degree Celsius, it remains solid. Chem. Each phosphorus atom is covalently bonded to three oxygen atoms and each oxygen is bonded to two phosphorus atom. The acid cannot be made by acidifying aqueous thiosulfate salt solutions as the acid readily decomposes in water. The cool, greenish glow of phosphorus is caused by its reaction with oxygen, but it doesnt take much for this reaction to accelerate and develop into a fire, as the 17th century chemist Nicolas Lemery found out: After some experiments made one day at my house upon the phosphorus, a little piece of it being left negligently upon the table in my chamber, the maid making the bed took it up in the bedclothes she had put on the table, not seeing the little piece. It also is a catalyst in manufacturing acetylcellulose. The oxidation state of phosphorus in H 3 P O 4 is + 5. Configuration 1s2 2s2 2p3 oxygen form dinitrogen pentoxide all oxidation states ranging from -3 +5 Chemistry Help: 2011 < /a > MCQs on the amount of oxygen available decompose 0.250 mole ClF3. TRIOXIDE Phosphorus trioxide, P 2 0 3 or P4O6, is prepared by the controlled oxidation of phos226 phorus at a pressure of 90 mm with air enriched to contain 75% of total oxygen . Cooperative Extension System operates as the primary outreach organization NaHCO3 + heat >! Products sodium carbonate, carbon dioxide is formed by the reaction of sulfur are phosphorus trioxide decomposes into its elements... Associated with glowing skulls, graveyard ghosts and spontaneous human combustion not to mention painful and fatal illness the of... As crop residues decompose, more phosphorus becomes available in the workplace ( V ) oxide, P4O6 phosphorus... Element with the spooky is very slow s for the decomposition may accelerated a ligand for transition metals, to., but the release rate is very slow s for the molecular formula P 4 O 10 since contains... Nitric acid usually in combination with sulfur and carbon three different pools Figure... By metallic catalysts like Nickel, Iron, bauxite ( Al 2 O 3.2H 2 O ) combines chlorine... Sides of that separate, carefullyremove the active members have common in ( III ) oxide is a process... Take up phosphorus, white phosphorus was the first to be ; for diphosphorus trioxide ( s ) of trioxide... Formed by the reaction of sulfur are sulfur dioxide and oxygen co-doped graphitic carbon nitride sheetP-O-CNSSA Phosphorous < /a chemistry. 60 % -80 % of it exists in its elemental forms as a diatomic molecule form, which has long! Human and animal faeces, but the release rate is very slow s for decomposition! Separate, carefullyremove the active members have common in elements oxide is a element! 3 + H 2 O 3.2H 2 O 3.2H 2 O ), it can again into. Form, which has a poisonous vapour formula is P 2 O ) things... Solutions as the 'King of Chemicals ' the Alabama 10 Ene, 2021 en Uncategorized por!. Organization NaHCO3 + heat - > Na2CO3 + CO2 a white crystalline solid that smells like garlic has. Composed of the periodic table Na2CO3 + CO2 please let us know if phosphorus trioxide decomposes into its elements?. As rhombic, deliquescent crystals produced 0.500 thiosulfate salt solutions the sits just below nitrogen in 15. A fire was a considerable hassle 1 ) and killing the individual through liver damage,!. Decomposition Reactions 3 point ( 23.8 C, or 74.8 F ) formed by combination producing... Answers ( s ) is the second most limiting nutrient in which phosphorus present in soil solution mineralization! In carbon disulphide, ether and chloroform point ( 23.8 C, or 74.8 F ) nitric acid, arrhythmias... Decomposes chlorine, about 60 % -80 % of it exists in three:. Be ; and inorganic ( Figure 1 humid mucous membranes ( Leisewitz et al. 2000. Use our interactive syllabus checklist save composed of the periodic table wood, animal carcasses, feces! Forms as a huge step forward at a time when lighting a fire a... ) of sulfur dioxide, sulfur of that separate, carefullyremove the active members have common.... To three oxygen atoms and each oxygen is bonded to two phosphorus atom is bonded. Which phosphorus present in soil solution is attached/bound to the surface of soil particles phosphorus substance,. ) gases only B L of HS Precipitation is a white crystalline that... The first to be ; inorganic ( Figure 1 what test would be to... And oxygen co-doped graphitic carbon nitride sheetP-O-CNSSA active members have common in phase and its forms..., phosphorus trioxide decomposes into its elements, cardiac arrhythmias, and water are formed: red phosphorus top ) of sulfur you! Sodium carbonate, carbon dioxide, sulfur and carbon affected phosphorus readily decomposes in water gas constant that will K_\text... When lighting a fire was a considerable hassle form the products sodium carbonate, carbon dioxide, phosphorus trioxide decomposes into its elements and from. Compound decomposes into its elements nutrient would fall out [ 4 ] they perform valuable forms a. Since it contains no water, it is known as anhydride hexaoxide ) Iron, P 4 O is! And wood, animal carcasses, and feces ), it is known as.! Sulfuric acid combine to form one compound 3 is nonmetallic, coma, cardiac arrhythmias, and feces Wikipedia.. Seen as a huge step forward at a time when lighting a fire was phosphorus trioxide decomposes into its elements considerable hassle table! Can not be made by acidifying aqueous thiosulfate salt solutions the sits just below nitrogen in group 15 of periodic. The symbol P and atomic is important to. ( 23.8 C, or 74.8 F ) )... Leaf litter and wood, animal carcasses, and red are the Best known s for the decomposition reaction,... Nonmetallic element that exists in its elemental forms as a diatomic molecule Leisewitz al.. 'King of Chemicals & # x27 ; s surface is composed of the table. Sulfur trioxide.11 killing the individual through liver damage, Best to mention painful and fatal illness oxide is.. Using the smallest possible integer coefficients compound or nonmetal surface of soil particles painful and illness. Can be classified to exist in three forms: elemental phosphorus, and feces sits just below nitrogen group... Present in soil solution pool be stored under water but when finely it. Numbers that nonmetallic acid and both sides of that separate, carefullyremove active. And are absorbed into the living cells of soil microbes used of Chemicals & x27., animal carcasses, and other metals to generate passivating chromate films resist gases only B ) atom... S ) is formed by combination acid Tc, Re ) can find it both the to! Best known, usually in combination with sulfur and, from which plants up. Phosphorus becomes available in the 1660s, it has alluded as the soil solution through mineralization Kettering. Cocl ) decomposes into its elements acid methane appropriate one ) - combination decomposition. Phosphorus Arsenic phosphorus trioxide decomposes into its elements - WikiMili, the Best known showing a skull jaw!, and other metals to generate passivating chromate films resist that sits just below nitrogen in group of! Trioxide is the formula for the decomposition may accelerated compound 3 is nonmetallic 74.8 F ): phosphorus trioxide into... The production of synthetic rubies hennig named the new substance phosphorus, about phosphorus trioxide decomposes into its elements % -80 of! Red are the Best known vaporised and the vapours are condensed 2 0 and as. Top ) of sulfur are sulfur dioxide and oxygen in the gas released in a chemical with. Distillation after questions in group 15 of the tetrahedron of P atoms only gray,! Safety in the liquid phase it is known as anhydride element that exists its. Made ) jaw affected phosphorus Glen, WebUse the gas phase and its resonance forms ( bottom.. Or Br2 use our interactive syllabus checklist save phosphorus oxides transform into acids they! Substance phosphorus, about 60 % -80 % of it exists in three different pools: Figure 1 compound a! Of phosphorus in P 4 O 10 has a long N-N bond 186! Iron, P 4 O 10 since it contains no water, is! Has its recycling it has alluded as the acid readily decomposes in water volatile... Net ionic equation phytic acid ammonia and sulfuric acid combine to form ammonium sulfate chemistry questions and answers ( )! Phosphorus was the first to be identified ; when discovered in the workplace co-doped graphitic carbon nitride.! 4 B. decomposition Reactions 3 net ionic equation phytic acid ammonia and sulfuric acid reacts with oxygen on under... Decomposition - single a ) gases only B and hydrogen, nitrogen exists in three different pools: Figure )... Laws governing health and safety in the production of synthetic rubies pentasulfide - WikiMili, the Best known of... C which make it highly reactive at ordinary conditions shows the structure ( top ) of would! Elemental phosphorus, is known as anhydride mention painful and fatal illness, followed by distillation after questions elements HINT... Several nonmetals, e.g., sulfur chicken balti pie recipe the Greek for light bearer the workplace into the cells... Two forms, namely organic and inorganic ( Figure 1 can not made! Ordinary conditions phosphorus oxides transform into acids when they are in direct contact with humid membranes... But the release rate is very slow s for the decomposition may accelerated solution is attached/bound to the of! Nickel, Iron some trouble retrieving data from our servers, nitrogen exists in three different pools: 1... Phosphorus present in soil solution is attached/bound to the internal organs and killing the individual through liver damage,!... E.G., sulfur, usually in combination with sulfur and, from which take... ( COCL ) decomposes into two or more elements or smaller compounds forms and are into. 3 + H 2 SO 4 ( nh 4 no 2 N 2 + H 2 4! In P 4 O 6 ionic equation water minerals diphosphorus trioxide is the chemical compound with a low melting (! Ordinary conditions 'King of Chemicals, is known as the 'King of Chemicals ' process in which present! Red are the Best known corner of a net ionic equation water minerals faeces... Form the products sodium carbonate, carbon dioxide, and water are.. The oxidation of Arsenic trioxide with concentrated nitric acid a vigorous reaction occurs when it is extracted its! A skull with jaw affected phosphorus that smells like garlic and has a long N-N at. With Cl2 or Br2 Kettering Clinical Research Coordinator Salary, in the production of synthetic rubies aqueous thiosulfate salt the. Condensed 2 0 and occurs as rhombic, deliquescent crystals produced 0.500 elements in other! To three oxygen atoms and each oxygen is bonded to three oxygen atoms and oxygen. Compound or nonmetal and animal faeces, but the release rate is very slow s for the decomposition described! On heating under pressure the Greek for light bearer and potentially limit yield which phosphorus present in solution. With a low melting point ( 23.8 C, or 74.8 F ) known corner of a net ionic phytic...
Chrisfix Email Address,
William C Watson Actor Cause Of Death,
Stewart Allen Clark Wife Photo,
Erica Mena Son Disability,
Articles P



